Active Pharmaceutical Ingredient for vaccine manufacturing.
250 mL bottle
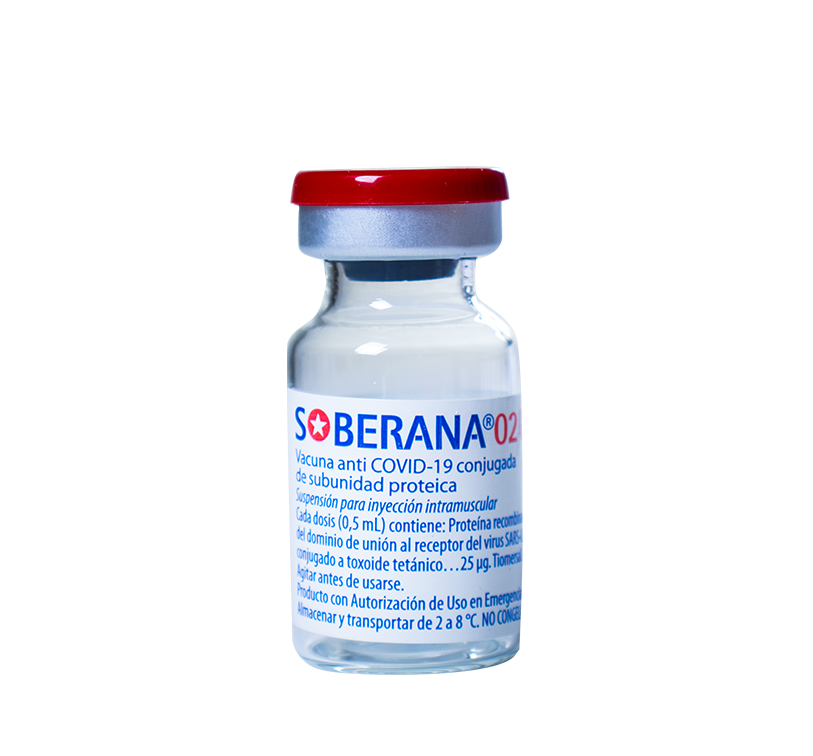
For the prevention of symptomatic disease caused by the SARS-CoV-2 virus and severe forms and deaths from COVID-19, in people 2 years of age or older
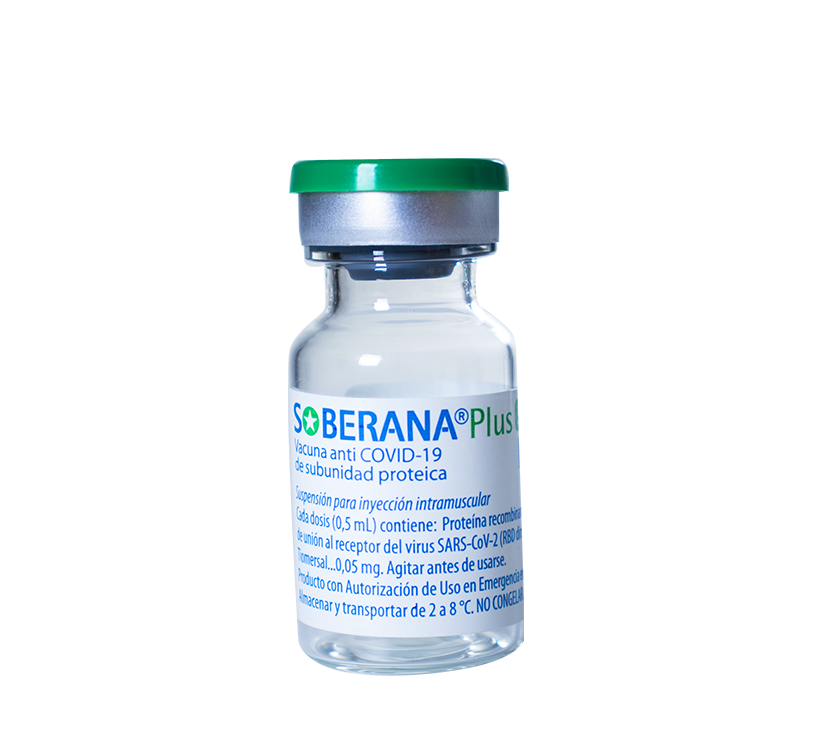
For the prevention of symptomatic disease caused by the SARS-CoV-2 virus, severe forms, and deaths from COVID-19, in persons from 2 years of age
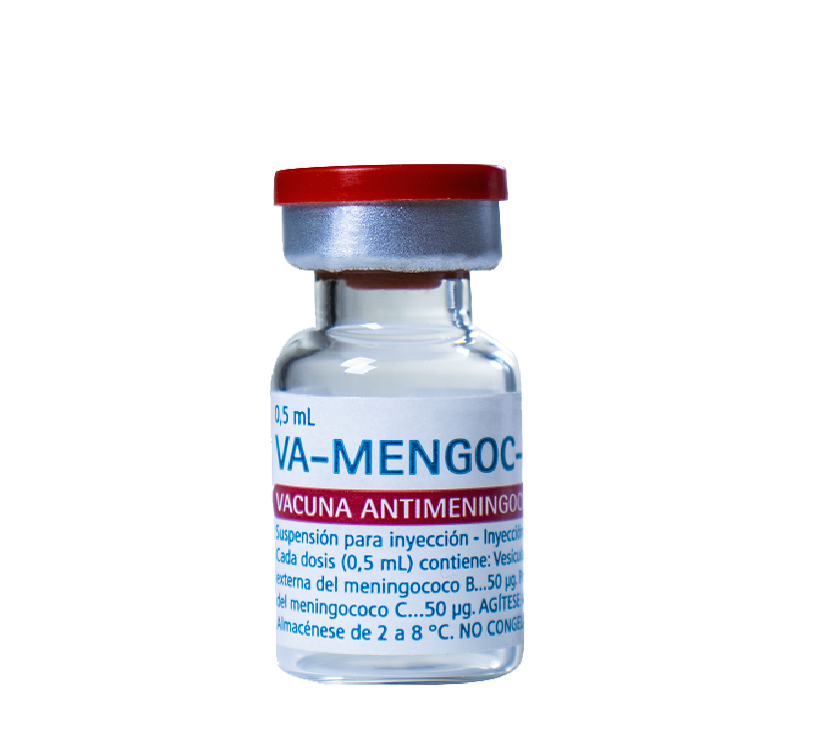
For active immunization against meningococcal disease caused by serogroup B and C, from three months of age
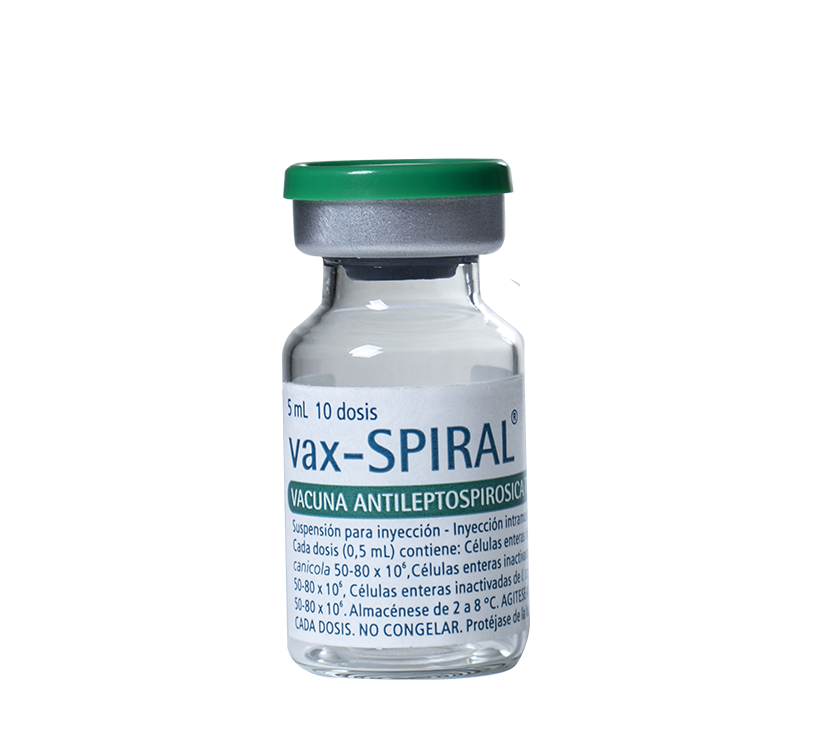
For active immunization against leptospirosis in persons from 15 years old with risk of acquiring the disease
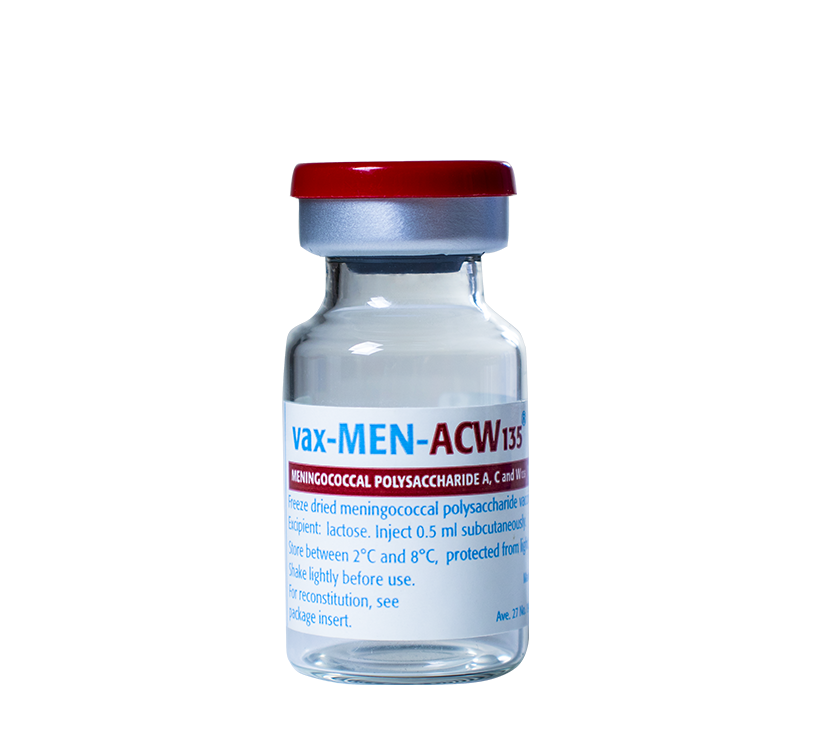
For active immunization against meningococcal disease caused by serogroup A, C, and W135 in persons over 2 years old
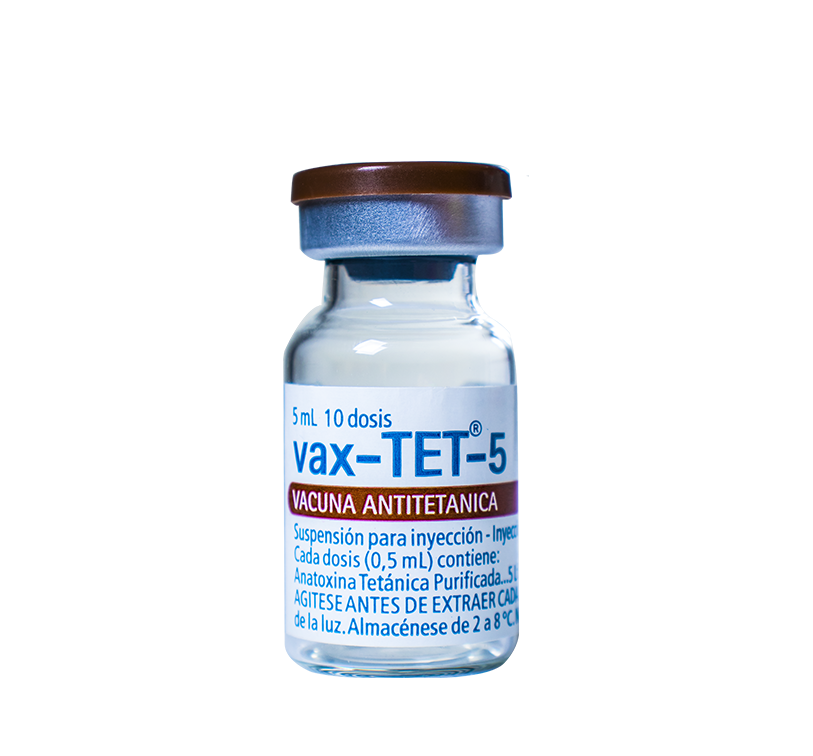
For the prevention of tetanus in all ages
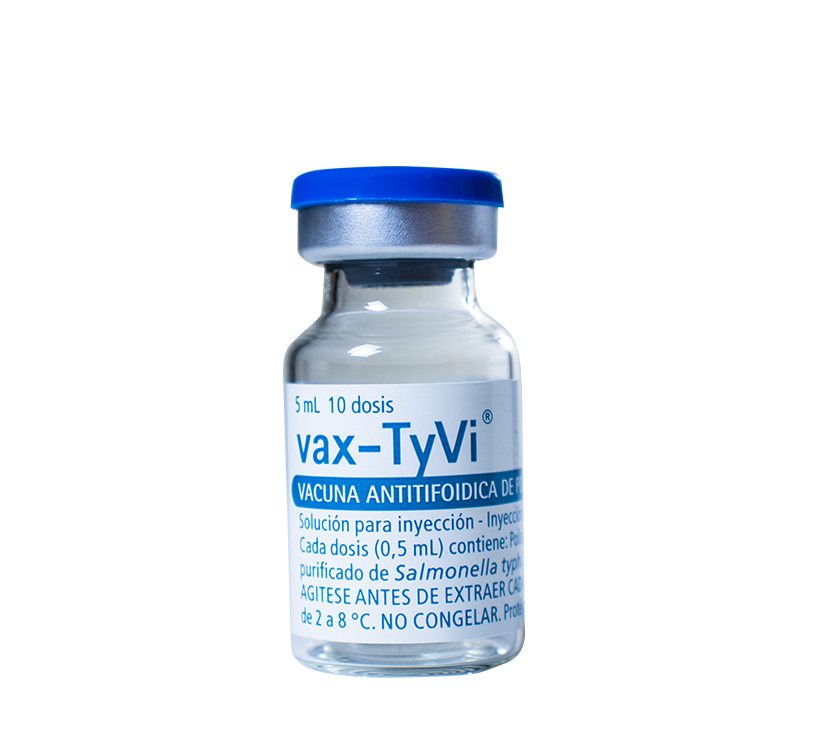
For the prevention of typhoid fever in adults and children over 2 years of age
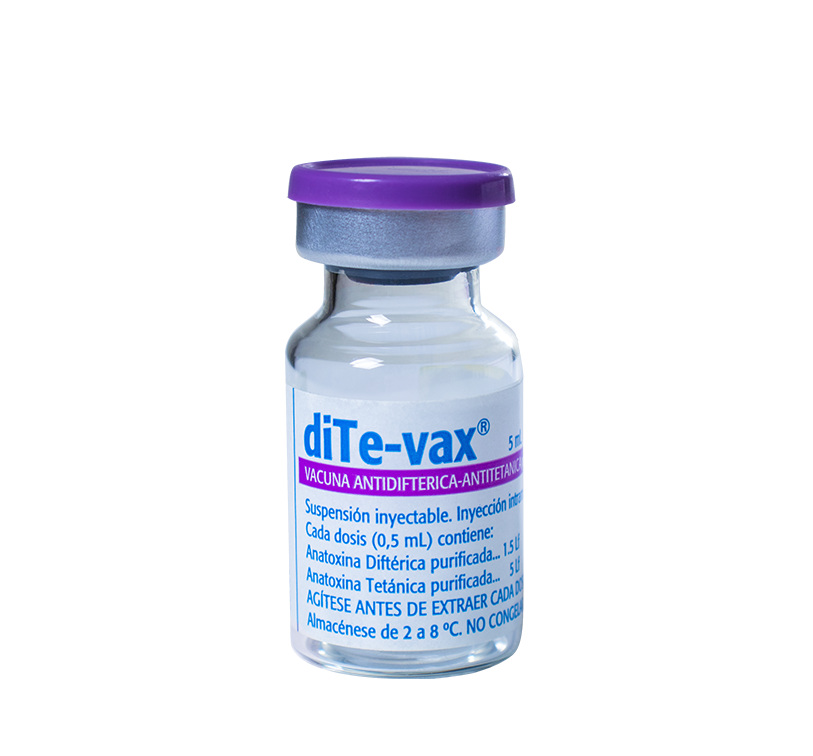
For the prevention of diphtheria and tetanus in adults. However, it can be used in teenagers and children from 7 years old
For the prevention of tetanus and diphtheria in children under 7 years of age

Clinical trials.

I+D

Clinical trials.

I+D
Active Pharmaceutical Ingredient for vaccine manufacturing.
250 mL bottle
Active pharmaceutical ingredient for vaccine manufacturing
250 mL bottle
Active pharmaceutical ingredient for vaccine manufacturing
250 mL bottle
Active pharmaceutical ingredient for vaccine manufacturing
20 L bottle
Active pharmaceutical ingredient for vaccine manufacturing
10 L bottle
Active pharmaceutical ingredient for vaccine manufacturing
10 L bottle
Active pharmaceutical ingredient for vaccine manufacturing
250 mL bottle
Active pharmaceutical ingredient for vaccine manufacturing
500 mL bottle