Ingrediente farmacéutico activo para la fabricación de vacunas.
Frasco 10 L
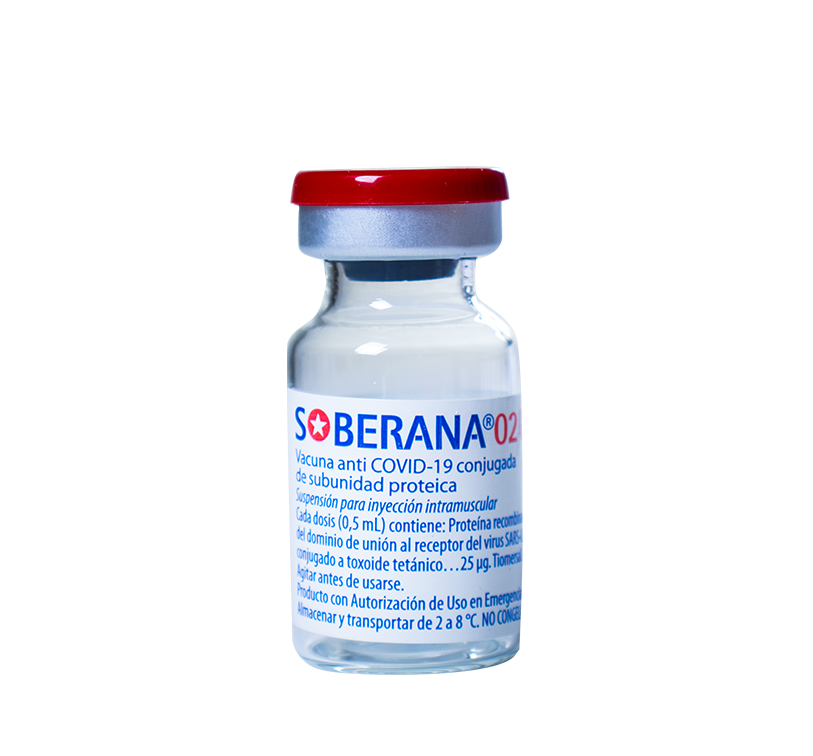
Suspension for intramuscular injection
Vials containing 10 dose, packaged in multiple boxes of 25 units
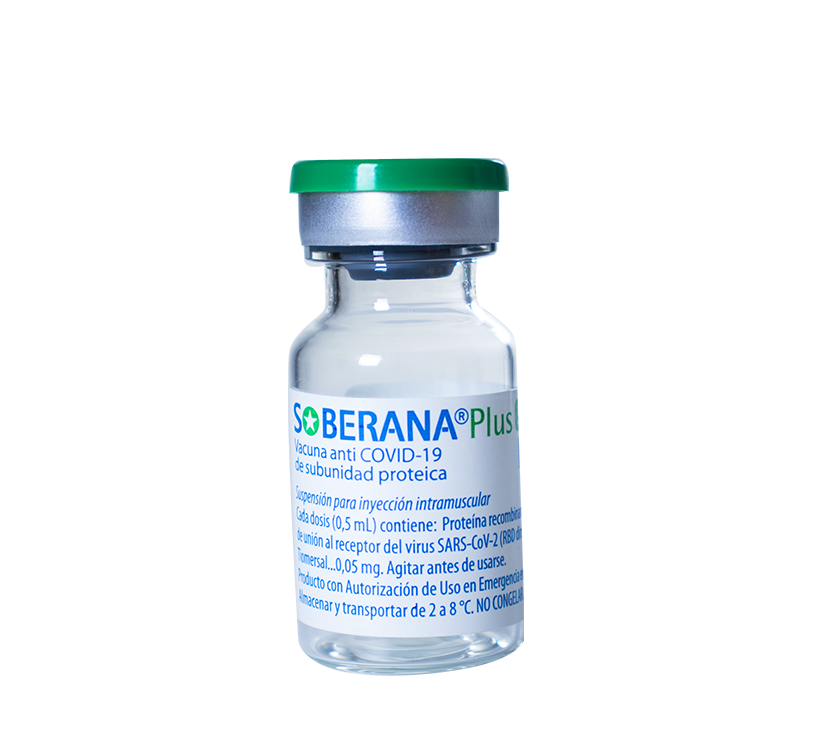
Suspension for intramuscular injection
Vials containing 1 dose, packaged in multiple boxes of 25 units
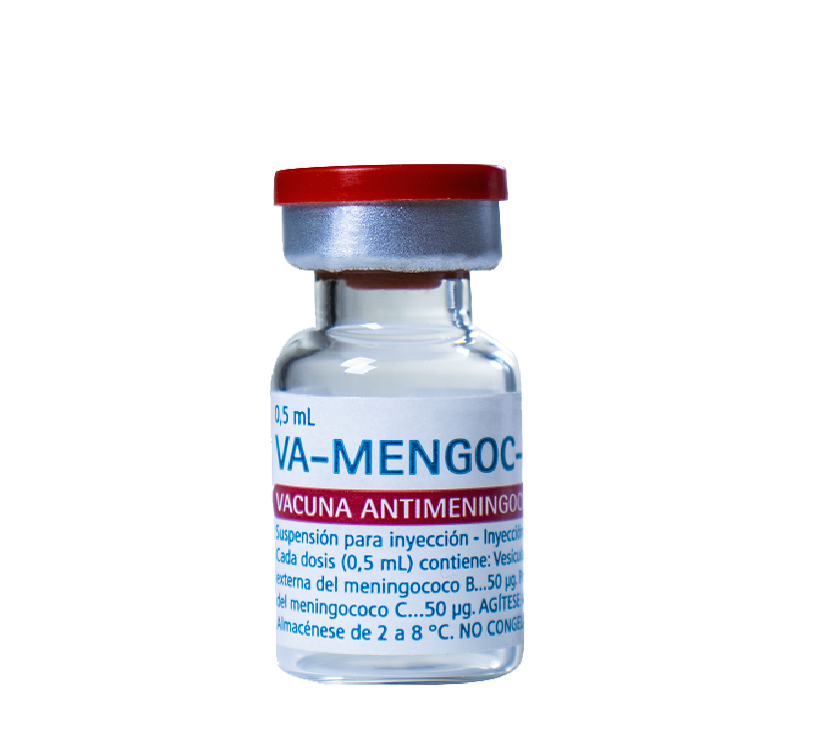
Suspension for intramuscular injection
Vials containing 1 dose, packaged in multiple boxes of 10 units
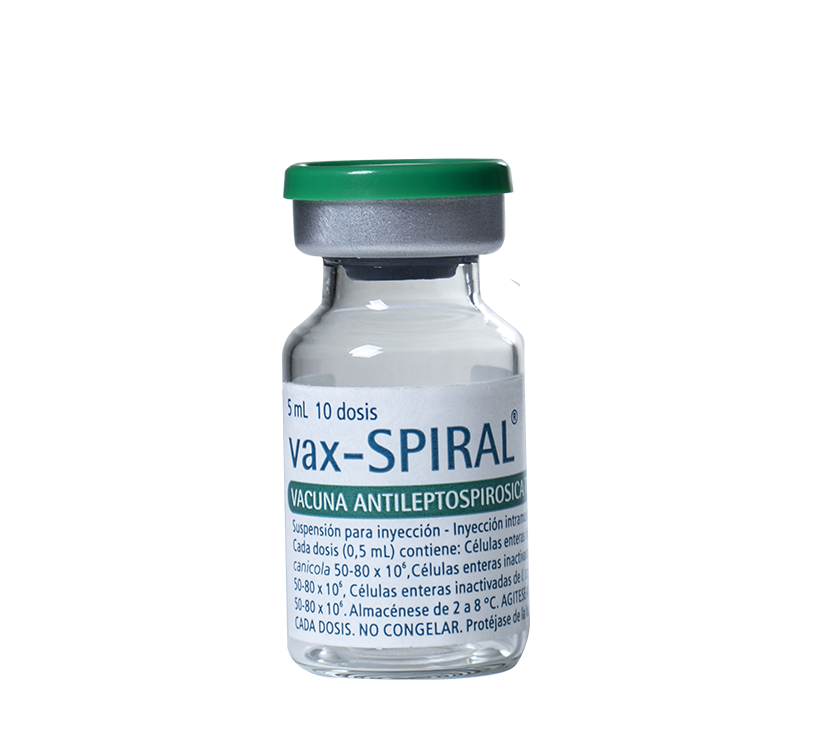
Suspension for intramuscular injection
Viales conteniendo 10 dosis, envasados en cajas múltiples de 10 unidades
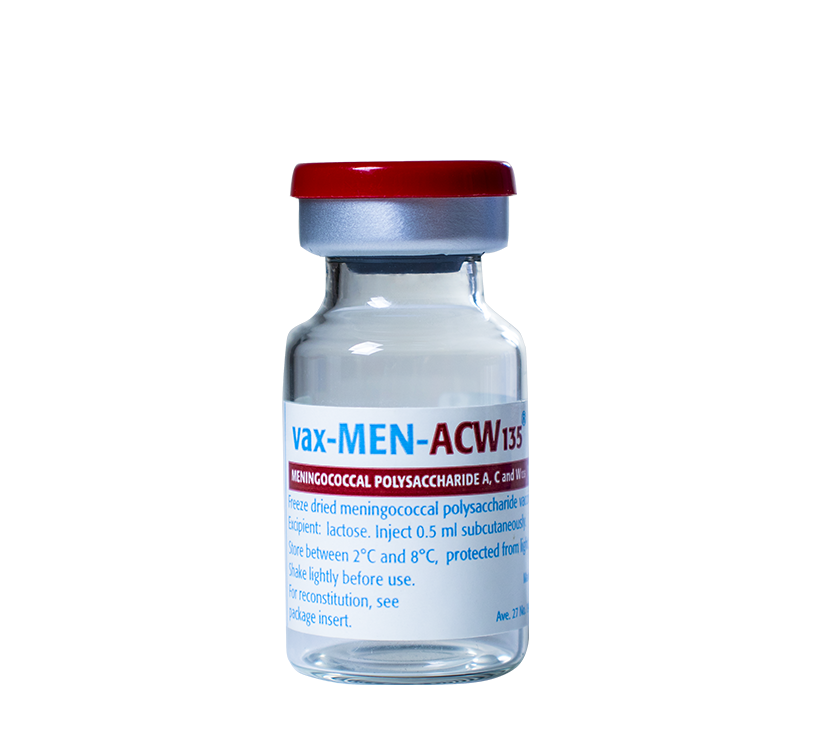
Lyophilized for subcutaneous injection
Vials containing 10 doses of the lyophilized vaccine and ampoule containing 5 mL of diluent
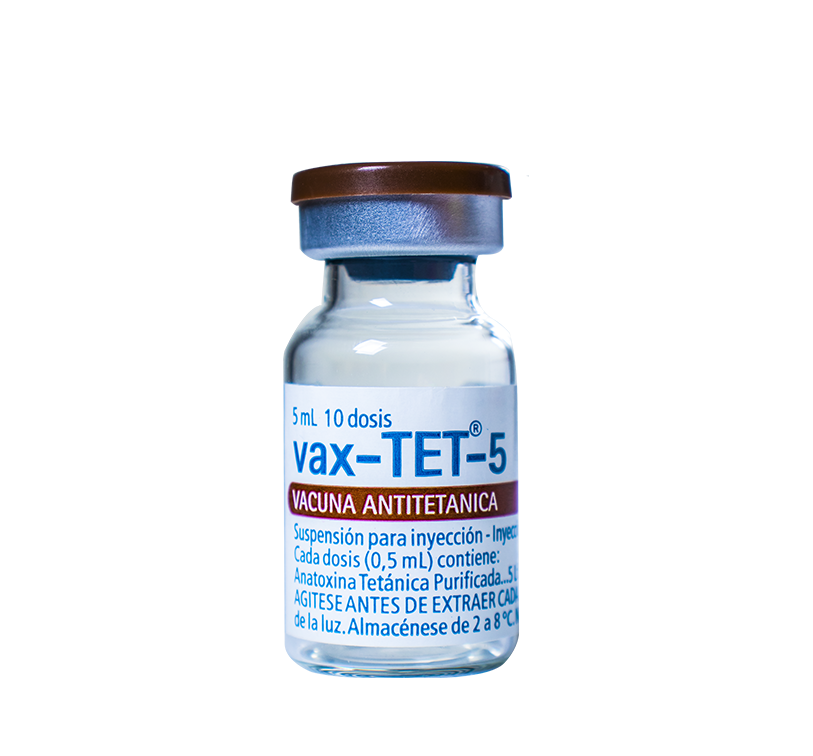
Vials containing 10 doses, packaged in multiple boxes of 10 units
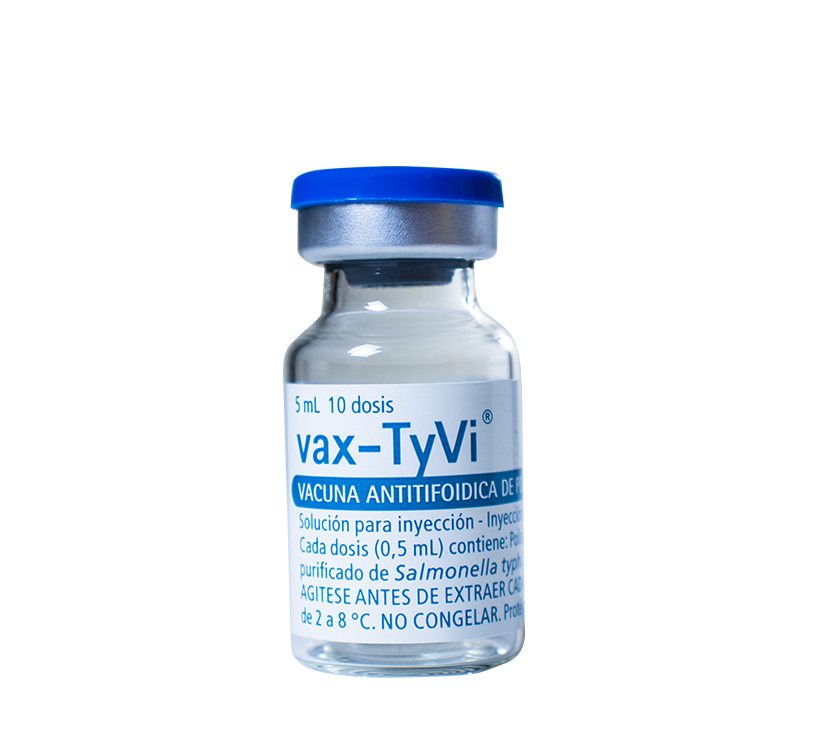
Vials containing 10 doses, packaged in multiple boxes of 10 units
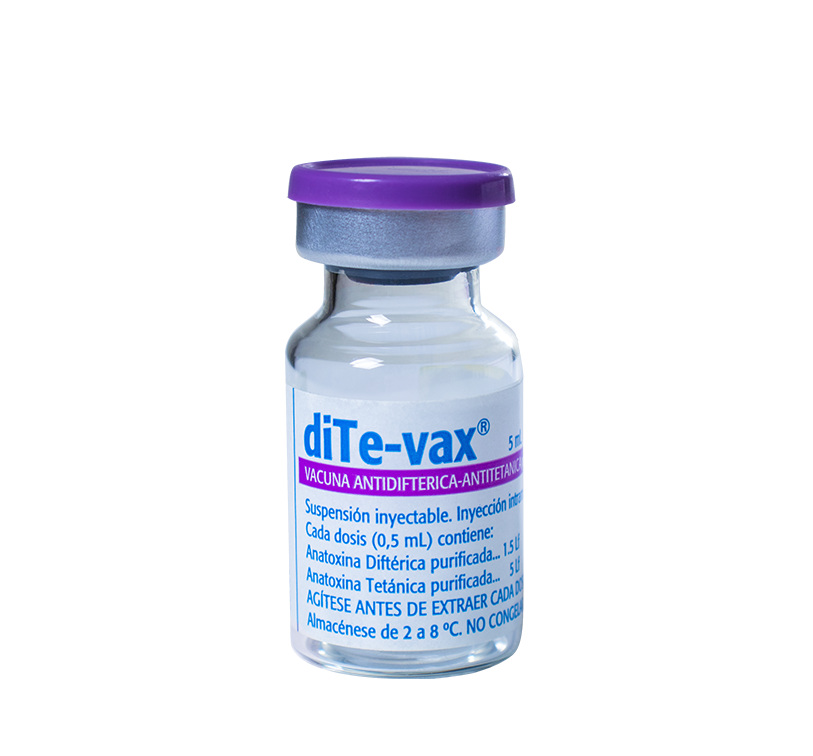
Suspension for intramuscular injection
Vials containing 10 doses, packaged in multiple boxes of 10 units
Suspension for intramuscular injection
Vials containing 10 doses, packaged in multiple boxes of 10 units
Ingrediente farmacéutico activo para la fabricación de vacunas.
Frasco 10 L
Ingrediente farmacéutico activo para la fabricación de vacunas.
Frasco 10 L
Ingrediente farmacéutico activo para la fabricación de vacunas.
Frasco 20 L
Ingrediente farmacéutico activo para la fabricación de vacunas.
Frasco 250 mL
Ingrediente farmacéutico activo para la fabricación de vacunas.
Frasco 250 mL
Ingrediente farmacéutico activo para la fabricación de vacunas.
Frasco 250 mL
Ingrediente farmacéutico activo para la fabricación de vacunas.
Frasco 250 mL
Ingrediente farmacéutico activo para la fabricación de vacunas.
Frasco 500 mL